Epiodyne has assembled an unparalleled team and technologies to solve the historical problems that have accompanied the use of opioids
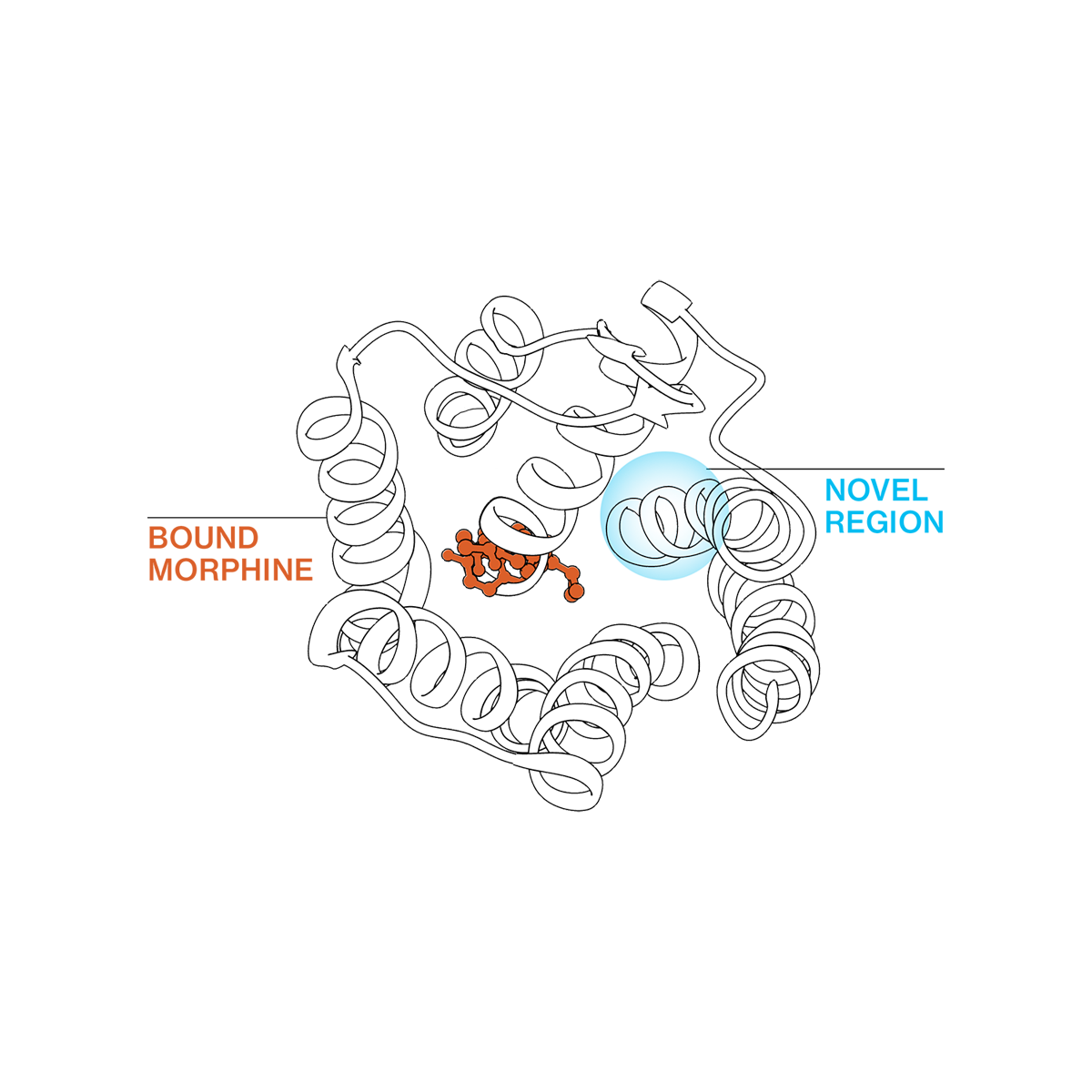
Based on groundbreaking insights into opioid receptor structure and activation, our team has overcome the challenges to selectively turning the MOR “on” and “off,” enabling the development of opioids without the side effects.
Our proprietary platform enables us to “tune” MOR small-molecule drug candidates to optimize efficacy while limiting liabilities
Built on our team’s deep understanding of mu opioid receptor structure, binding, and signaling activation, our platform enables the development of a full spectrum of precisely calibrated small-molecule mu opioid receptor (MOR) modulators that are tunable for desired therapeutic outcomes—from full inhibitors to full agonists.
Generating opioid therapeutics with specifically calibrated functions
Our platform is applicable to creating optimized MOR modulators for treating pain, opioid addiction, depression, OCD, and other mental illnesses.
Our lead program, EPD 2520, is a partial agonist of the mu opoid receptor designed to effectively relieve pain and opioid dependency with less potential for addiction and respiratory depression. In preclinical studies, our lead candidate, EPD-2520 demonstrated:
Effective Pain Management & Improved Safety
- Analgesia equal to current opioid therapies: EPD-2520 was as effective as doses of oxycodone associated with mortalities in preclinical studies
- Minimal to no respiratory liability: EPD-2520 was not associated with lethality. More importantly, EPD-2520 did not lead to respiratory depression in normoxic and hypoxic conditions, an unfavorable liability of oxycodone and fentanyl
- Reduced addiction potential: Unlike oxycodone, EPD-2520 did not result in drug seeking behavior over a wide dose range
Relieve Opioid Dependency – “A Better Buprenorphine”
- Buprenorphine-like intrinsic efficacy to promote compliance and reduce risks: EPD-2520’s activity is designed to eliminate cravings, but not enough to restore addictive behavior
- Reduced cravings: During detoxification from morphine, EPD-2520 reduced cravings at a similar level to buprenorphine
- Reduced dependence: Buprenorphine is known to precipitate opioid withdrawal during induction. EPD-2520 showed significantly reduced precipitated withdrawal in Naltrexone treated animals compared to buprenorphine
- Reduced addiction potential: Animals did not demonstrate drug seeking behavior as they had for buprenorphine
EPD-2520 is the only drug designed to offer the unique combination of effective pain relief with reduced side effects and addiction potential.

PUBLICATIONS
Structure of the nanobody-stabilized active state of the kappa opioid receptor. Che T, Majumdar S, Zaidi SA, Ondachi P, McCorvy JD, Wang S, Mosier PD, Uprety R, Vardy E, Krumm BE, Han GW, Lee MY, Pardon E, Steyaert J, Huang XP, Strachan RT, Tribo AR, Pasternak GW, Carroll FI, Stevens RC, Cherezov V, Katritch V, Wacker D, Roth BL. Cell. 2018 January 11; 172(1-2): 55-67.
Structure-based discovery of opioid analgesics with reduced side effects. Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang XP, Sassano MF, Giguère PM, Löber S, Duan D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK. Nature. 2016 August 17; 537: 185–190.
Structural insights into µ-opioid receptor activation. Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, Kato HE, Livingston KE, Thorsen TS, Kling RC, Granier S, Gmeiner P, Husbands SM, Traynor JR, Weis WI, Steyaert J, Dror RO, Kobilka BK. Nature. 2015 August 5; 524: 315–321.
Molecular control of δ-opioid receptor signalling. Fenalti G, Giguere P, Katritch V, Huang XP, Thompson AA, Cherezov V, Roth BL, Stevens RC. Nature. 2014 January 12; 506: 191–196.
Structure of the δ-opioid receptor bound to naltrindole. Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Nature. 2012 May 16; 485(7398):400-4.
Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Nature. 2012 March 21; 485(7398): 321-6.
Structure of the human κ-opioid receptor in complex with JDTic. Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Nature. 2012 March 21; 485(7398): 327-32.